What Does The Microtubule Do In An Animal Cell

Microtubule and tubulin metrics[ane]
Microtubules are polymers of tubulin that class part of the cytoskeleton and provide construction and shape to eukaryotic cells. Microtubules tin can be equally long every bit 50 micrometres, every bit wide equally 23 to 27 nm[2] and have an inner diameter between 11 and 15 nm.[3] They are formed by the polymerization of a dimer of two globular proteins, blastoff and beta tubulin into protofilaments that can then associate laterally to form a hollow tube, the microtubule.[4] The most common form of a microtubule consists of 13 protofilaments in the tubular organization.

Microtubules are one of the cytoskeletal filament systems in eukaryotic cells. The microtubule cytoskeleton is involved in the transport of cloth within cells, carried out by motor proteins that move on the surface of the microtubule.
Microtubules play an important role in a number of cellular processes. They are involved in maintaining the structure of the jail cell and, together with microfilaments and intermediate filaments, they course the cytoskeleton. They also make up the internal construction of cilia and flagella. They provide platforms for intracellular transport and are involved in a diversity of cellular processes, including the motility of secretory vesicles, organelles, and intracellular macromolecular assemblies.[5] They are likewise involved in cell division (past mitosis and meiosis) and are the main constituents of mitotic spindles, which are used to pull eukaryotic chromosomes autonomously.
Microtubules are nucleated and organized by microtubule-organizing centres, such as the centrosome found in the center of many animal cells or the basal bodies of cilia and flagella, or the spindle pole bodies found in near fungi.
At that place are many proteins that bind to microtubules, including the motor proteins dynein and kinesin, microtubule-severing proteins like katanin, and other proteins of import for regulating microtubule dynamics.[half dozen] Recently an actin-like protein has been found in the gram-positive bacterium Bacillus thuringiensis, which forms a microtubule-like construction chosen a nanotubule, involved in plasmid segregation.[7] Other bacterial microtubules have a ring of five protofilaments.
History [edit]
Tubulin and microtubule-mediated processes, like prison cell locomotion, were seen by early microscopists, similar Leeuwenhoek (1677). Nonetheless, the gristly nature of flagella and other structures were discovered 2 centuries later, with improved light microscopes, and confirmed in the 20th century with the electron microscope and biochemical studies.[eight]
In vitro assays for microtubule motor proteins such as dynein and kinesin are researched by fluorescently tagging a microtubule and fixing either the microtubule or motor proteins to a microscope slide, then visualizing the slide with video-enhanced microscopy to record the travel of the motor proteins. This allows the move of the motor proteins along the microtubule or the microtubule moving across the motor proteins.[nine] Consequently, some microtubule processes can exist determined by kymograph.[ten]
Structure [edit]

Drawing representation of the structure of α(yellowish)/β(red)-tubulin heterodimer, GTP and Gross domestic product.[11]
In eukaryotes, microtubules are long, hollow cylinders made up of polymerised α- and β-tubulin dimers.[12] The inner space of the hollow microtubule cylinders is referred to equally the lumen. The α and β-tubulin subunits are ~50% identical at the amino acid level, and both take a molecular weight of approximately 50 kDa.[thirteen] [xiv]
These α/β-tubulin dimers polymerize end-to-stop into linear protofilaments that associate laterally to form a unmarried microtubule, which can then be extended by the addition of more than α/β-tubulin dimers. Typically, microtubules are formed past the parallel association of thirteen protofilaments, although microtubules composed of fewer or more protofilaments take been observed in diverse species[fifteen] besides as in vitro.[xvi]
Microtubules have a singled-out polarity that is disquisitional for their biological function. Tubulin polymerizes finish to end, with the β-subunits of one tubulin dimer contacting the α-subunits of the next dimer. Therefore, in a protofilament, 1 end volition have the α-subunits exposed while the other end will have the β-subunits exposed. These ends are designated the (−) and (+) ends, respectively. The protofilaments parcel parallel to one some other with the same polarity, and then, in a microtubule, there is one end, the (+) cease, with but β-subunits exposed, while the other end, the (−) end, has only α-subunits exposed. While microtubule elongation can occur at both the (+) and (−) ends, it is significantly more rapid at the (+) terminate.[17]
The lateral clan of the protofilaments generates a pseudo-helical structure, with one turn of the helix containing 13 tubulin dimers, each from a different protofilament. In the most mutual "13-3" architecture, the 13th tubulin dimer interacts with the next tubulin dimer with a vertical offset of 3 tubulin monomers due to the helicity of the turn. There are other alternative architectures, such as xi-iii, 12-3, fourteen-3, 15-four, or sixteen-4, that accept been detected at a much lower occurrence.[18] Microtubules can likewise morph into other forms such as helical filaments, which are observed in protist organisms like foraminifera.[19] There are two singled-out types of interactions that can occur between the subunits of lateral protofilaments within the microtubule called the A-blazon and B-type lattices. In the A-type lattice, the lateral associations of protofilaments occur between next α and β-tubulin subunits (i.e. an α-tubulin subunit from ane protofilament interacts with a β-tubulin subunit from an adjacent protofilament). In the B-type lattice, the α and β-tubulin subunits from one protofilament interact with the α and β-tubulin subunits from an adjacent protofilament, respectively. Experimental studies have shown that the B-type lattice is the master arrangement inside microtubules. However, in most microtubules there is a seam in which tubulin subunits interact α-β.[xx]
The sequence and exact composition of molecules during microtubule formation can thus exist summarised equally follows: A β-tubulin connects in the context of a non-real covalent bond with an α-tubulin, which in continued form are a heterodimer, since they consist of two different polypeptides (β-tubulin and α-tubulin). So after the heterodimers are formed, they join together to form long chains that ascent figuratively in i management (e.grand. upwards). These heterodimers, which are connected in a certain direction, form protofilaments. These long chains (protofilaments) now gradually accumulate side by side to each other so that a tube-like construction is formed, which has a lumen typical of a tube. Accordingly, mostly 13 protofilaments class the outer wall of the microtubules. It is besides of import to notation that the heterodimers consist of a positive and negative end, with alpha-tubulin forming the negative finish and beta-tubulin the positive end. Due to the fact that the heterodimers are stacked on superlative of each other, there is always a negative and positive end. Microtubules grow by an add-on of heterodimers at the plus end.
Some species of Prosthecobacter also contain microtubules. The structure of these bacterial microtubules is similar to that of eukaryotic microtubules, consisting of a hollow tube of protofilaments assembled from heterodimers of bacterial tubulin A (BtubA) and bacterial tubulin B (BtubB). Both BtubA and BtubB share features of both α- and β-tubulin. Dissimilar eukaryotic microtubules, bacterial microtubules exercise not require chaperones to fold.[21] In dissimilarity to the 13 protofilaments of eukaryotic microtubules, bacterial microtubules comprise but five.[22]
Intracellular organization [edit]
Microtubules are part of the cytoskeleton, a structural network within the prison cell'south cytoplasm. The roles of the microtubule cytoskeleton include mechanical support, organization of the cytoplasm, send, motility and chromosome segregation. In developing neurons microtubules are known as neurotubules,[23] and they tin can modulate the dynamics of actin, some other component of the cytoskeleton.[24] A microtubule is capable of growing and shrinking in club to generate force, and in that location are motor proteins that allow organelles and other cellular components to be carried along a microtubule. This combination of roles makes microtubules important for organizing and moving intracellular constituents.
The organization of microtubules in the prison cell is cell-blazon specific. In epithelia, the minus-ends of the microtubule polymer are anchored near the site of cell-cell contact and organized forth the apical-basal axis. After nucleation, the minus-ends are released and then re-anchored in the periphery by factors such as ninein and PLEKHA7.[25] In this manner, they can facilitate the transport of proteins, vesicles and organelles along the apical-basal axis of the jail cell. In fibroblasts and other mesenchymal cell-types, microtubules are anchored at the centrosome and radiate with their plus-ends outwards towards the jail cell periphery (as shown in the first effigy). In these cells, the microtubules play of import roles in cell migration. Moreover, the polarity of microtubules is acted upon by motor proteins, which organize many components of the cell, including the endoplasmic reticulum and the Golgi apparatus.

Components of the eukaryotic cytoskeleton. Actin filaments are shown in cherry-red, microtubules are in green, and the nuclei are in blue. The cystoskeleton provides the jail cell with an inner framework and enables information technology to movement and change shape.
Microtubule polymerization [edit]
Nucleation [edit]
Nucleation is the event that initiates the formation of microtubules from the tubulin dimer. Microtubules are typically nucleated and organized by organelles called microtubule-organizing centres (MTOCs). Independent within the MTOC is another blazon of tubulin, γ-tubulin, which is singled-out from the α- and β-subunits of the microtubules themselves. The γ-tubulin combines with several other associated proteins to form a lock washer-like structure known as the "γ-tubulin ring complex" (γ-TuRC). This complex acts every bit a template for α/β-tubulin dimers to begin polymerization; it acts as a cap of the (−) finish while microtubule growth continues away from the MTOC in the (+) direction.[26]
The centrosome is the principal MTOC of almost prison cell types. Notwithstanding, microtubules tin be nucleated from other sites as well. For case, cilia and flagella have MTOCs at their base termed basal bodies. In addition, work from the Kaverina group at Vanderbilt, also as others, suggests that the Golgi apparatus tin serve as an important platform for the nucleation of microtubules.[27] Considering nucleation from the centrosome is inherently symmetrical, Golgi-associated microtubule nucleation may allow the cell to establish asymmetry in the microtubule network. In recent studies, the Vale group at UCSF identified the poly peptide complex augmin as a disquisitional factor for centrosome-dependent, spindle-based microtubule generation. Information technology that has been shown to interact with γ-TuRC and increase microtubule density around the mitotic spindle origin.[28]
Some jail cell types, such equally plant cells, do not comprise well defined MTOCs. In these cells, microtubules are nucleated from discrete sites in the cytoplasm. Other prison cell types, such as trypanosomatid parasites, have a MTOC but it is permanently institute at the base of a flagellum. Here, nucleation of microtubules for structural roles and for generation of the mitotic spindle is not from a approved centriole-like MTOC.
Polymerization [edit]
Following the initial nucleation effect, tubulin monomers must be added to the growing polymer. The process of calculation or removing monomers depends on the concentration of αβ-tubulin dimers in solution in relation to the critical concentration, which is the steady state concentration of dimers at which there is no longer whatsoever internet associates or disassembly at the end of the microtubule. If the dimer concentration is greater than the critical concentration, the microtubule will polymerize and abound. If the concentration is less than the critical concentration, the length of the microtubule will decrease.[29]
Microtubule dynamics [edit]
Dynamic instability [edit]
Animation of the microtubule dynamic instability. Tubulin dimers leap to GTP (scarlet) bind to the growing terminate of a microtubule and afterwards hydrolyze GTP into Gross domestic product (blue).
Dynamic instability refers to the coexistence of assembly and disassembly at the ends of a microtubule. The microtubule tin dynamically switch betwixt growing and shrinking phases in this region.[30] Tubulin dimers tin bind two molecules of GTP, one of which can be hydrolyzed subsequent to assembly. During polymerization, the tubulin dimers are in the GTP-spring land.[12] The GTP spring to α-tubulin is stable and it plays a structural function in this jump state. However, the GTP bound to β-tubulin may be hydrolyzed to GDP shortly subsequently assembly. The assembly properties of Gross domestic product-tubulin are unlike from those of GTP-tubulin, as Gdp-tubulin is more prone to depolymerization.[31] A Gross domestic product-bound tubulin subunit at the tip of a microtubule will tend to autumn off, although a GDP-bound tubulin in the centre of a microtubule cannot spontaneously pop out of the polymer. Since tubulin adds onto the end of the microtubule in the GTP-spring state, a cap of GTP-leap tubulin is proposed to exist at the tip of the microtubule, protecting it from disassembly. When hydrolysis catches up to the tip of the microtubule, it begins a rapid depolymerization and shrinkage. This switch from growth to shrinking is called a catastrophe. GTP-bound tubulin can begin calculation to the tip of the microtubule again, providing a new cap and protecting the microtubule from shrinking. This is referred to every bit "rescue".[32]
"Search and capture" model [edit]
In 1986, Marc Kirschner and Tim Mitchison proposed that microtubules utilise their dynamic properties of growth and shrinkage at their plus ends to probe the 3 dimensional space of the cell. Plus ends that meet kinetochores or sites of polarity become captured and no longer brandish growth or shrinkage. In contrast to normal dynamic microtubules, which have a half-life of v–10 minutes, the captured microtubules can last for hours. This idea is commonly known equally the "search and capture" model.[33] Indeed, piece of work since and then has largely validated this thought. At the kinetochore, a variety of complexes accept been shown to capture microtubule (+)-ends.[34] Moreover, a (+)-end capping activity for interphase microtubules has likewise been described.[35] This later activity is mediated past formins,[36] the adenomatous polyposis coli protein, and EB1,[37] a protein that tracks along the growing plus ends of microtubules.
Regulation of microtubule dynamics [edit]
Postal service-translational modifications [edit]

Image of a fibroblast cell containing fluorescently labeled actin (cherry-red) and microtubules (light-green).
Although most microtubules have a one-half-life of v–ten minutes, certain microtubules tin can remain stable for hours.[35] These stabilized microtubules accrue post-translational modifications on their tubulin subunits past the activeness of microtubule-spring enzymes.[38] [39] However, in one case the microtubule depolymerizes, almost of these modifications are rapidly reversed by soluble enzymes. Since nearly modification reactions are slow while their opposite reactions are rapid, modified tubulin is only detected on long-lived stable microtubules. Most of these modifications occur on the C-terminal region of alpha-tubulin. This region, which is rich in negatively charged glutamate, forms relatively unstructured tails that projection out from the microtubule and form contacts with motors. Thus, it is believed that tubulin modifications regulate the interaction of motors with the microtubule. Since these stable modified microtubules are typically oriented towards the site of cell polarity in interphase cells, this subset of modified microtubules provide a specialized route that helps deliver vesicles to these polarized zones. These modifications include:
- Detyrosination: the removal of the C-terminal tyrosine from alpha-tubulin. This reaction exposes a glutamate at the new C-terminus. Equally a result, microtubules that accumulate this modification are ofttimes referred to equally Glu-microtubules. Although the tubulin carboxypeptidase has even so to be identified, the tubulin—tyrosine ligase (TTL) is known.[40]
- Delta2: the removal of the last ii residues from the C-terminus of alpha-tubulin.[41] Different detyrosination, this reaction is thought to be irreversible and has only been documented in neurons.
- Acetylation: the addition of an acetyl group to lysine 40 of alpha-tubulin. This modification occurs on a lysine that is accessible only from the inside of the microtubule, and it remains unclear how enzymes access the lysine remainder. The nature of the tubulin acetyltransferase remains controversial, but it has been institute that in mammals the major acetyltransferase is ATAT1.[42] however, the reverse reaction is known to exist catalyzed by HDAC6.[43]
- Polyglutamylation: the addition of a glutamate polymer (typically 4-half-dozen residues long[44]) to the gamma-carboxyl group of any one of v glutamates found well-nigh the stop of alpha-tubulin. Enzymes related to TTL add the initial branching glutamate (TTL4,5 and vii), while other enzymes that belong to the same family lengthen the polyglutamate concatenation (TTL6,eleven and 13).[39]
- Polyglycylation: the addition of a glycine polymer (2-10 residues long) to the gamma-carboxyl group of any i of five glutamates found nearly the finish of beta-tubulin. TTL3 and 8 add the initial branching glycine, while TTL10 lengthens the polyglycine chain.[39]
Tubulin is also known to be phosphorylated, ubiquitinated, sumoylated, and palmitoylated.[38]
Tubulin-bounden drugs and chemical effects [edit]
A broad diversity of drugs are able to bind to tubulin and modify its assembly properties. These drugs can have an event at intracellular concentrations much lower than that of tubulin. This interference with microtubule dynamics tin can take the effect of stopping a prison cell'due south cell bicycle and tin lead to programmed prison cell death or apoptosis. Notwithstanding, there are information to propose that interference of microtubule dynamics is bereft to block the cells undergoing mitosis.[45] These studies have demonstrated that suppression of dynamics occurs at concentrations lower than those needed to block mitosis. Suppression of microtubule dynamics by tubulin mutations or by drug handling accept been shown to inhibit cell migration.[46] Both microtubule stabilizers and destabilizers can suppress microtubule dynamics.
The drugs that tin alter microtubule dynamics include:
- The cancer-fighting taxane class of drugs (paclitaxel (taxol) and docetaxel) block dynamic instability by stabilizing GDP-bound tubulin in the microtubule. Thus, even when hydrolysis of GTP reaches the tip of the microtubule, in that location is no depolymerization and the microtubule does not shrink back.
Taxanes (alone or in combination with platinum derivatives (carboplatine) or gemcitabine) are used against breast and gynecological malignancies, squamous-prison cell carcinomas (head-and-neck cancers, some lung cancers), etc.
- The epothilones, due east.g. Ixabepilone, piece of work in a similar fashion to the taxanes.
- Vinorelbine, Nocodazole, vincristine, and colchicine take the contrary consequence, blocking the polymerization of tubulin into microtubules.
- Eribulin binds to the (+) growing terminate of the microtubules. Eribulin exerts its anticancer effects by triggering apoptosis of cancer cells following prolonged and irreversible mitotic blockade.
Expression of β3-tubulin has been reported to alter cellular responses to drug-induced suppression of microtubule dynamics. In general the dynamics are ordinarily suppressed by low, subtoxic concentrations of microtubule drugs that also inhibit jail cell migration. However, incorporating β3-tubulin into microtubules increases the concentration of drug that is needed to suppress dynamics and inhibit prison cell migration. Thus, tumors that limited β3-tubulin are not but resistant to the cytotoxic effects of microtubule targeted drugs, simply as well to their power to suppress tumor metastasis. Moreover, expression of β3-tubulin likewise counteracts the power of these drugs to inhibit angiogenesis which is commonly some other important facet of their action.[ commendation needed ]
Microtubule polymers are extremely sensitive to various ecology furnishings. Very low levels of free calcium can destabilize microtubules and this prevented early researchers from studying the polymer in vitro.[12] Cold temperatures also cause rapid depolymerization of microtubules. In contrast, heavy water promotes microtubule polymer stability.[47]
Proteins that interact with microtubules [edit]
Microtubule-associated proteins (MAPs) [edit]
MAPs have been shown to play a crucial role in the regulation of microtubule dynamics in-vivo. The rates of microtubule polymerization, depolymerization, and catastrophe vary depending on which microtubule-associated proteins (MAPs) are present. The originally identified MAPs from brain tissue can be classified into two groups based on their molecular weight. This start form comprises MAPs with a molecular weight below 55-62 kDa, and are called τ (tau) proteins. In-vitro, tau proteins have been shown to directly bind microtubules, promote nucleation and prevent disassembly, and to induce the formation of parallel arrays.[48] Additionally, tau proteins have also been shown to stabilize microtubules in axons and have been implicated in Alzheimer'southward disease.[49] The 2d class is composed of MAPs with a molecular weight of 200-yard kDa, of which there are four known types: MAP-one, MAP-two, MAP-three and MAP-4. MAP-1 proteins consists of a set of three dissimilar proteins: A, B and C. The C protein plays an important role in the retrograde transport of vesicles and is likewise known as cytoplasmic dynein. MAP-2 proteins are located in the dendrites and in the body of neurons, where they demark with other cytoskeletal filaments. The MAP-4 proteins are found in the majority of cells and stabilize microtubules. In addition to MAPs that accept a stabilizing effect on microtubule construction, other MAPs can have a destabilizing result either past cleaving or past inducing depolymerization of microtubules. Three proteins called katanin, spastin, and fidgetin accept been observed to regulate the number and length of microtubules via their destabilizing activities. Furthermore, KIAA1211L is predicted to be localized to the microtubules.[50]
Plus-finish tracking proteins (+TIPs) [edit]
Plus end tracking proteins are MAP proteins which bind to the tips of growing microtubules and play an important role in regulating microtubule dynamics. For example, +TIPs accept been observed to participate in the interactions of microtubules with chromosomes during mitosis. The first MAP to be identified as a +TIP was CLIP170 (cytoplasmic linker poly peptide), which has been shown to play a role in microtubule depolymerization rescue events. Additional examples of +TIPs include EB1, EB2, EB3, p150Glued, Dynamitin, Lis1, CLIP115, CLASP1, and CLASP2.[ citation needed ]
Motor proteins [edit]
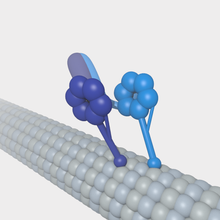
A cytoplasmic dynein motor jump to a microtubule.

A kinesin molecule bound to a microtubule.
Microtubules can act every bit substrates for motor proteins that are involved in of import cellular functions such as vesicle trafficking and cell division. Unlike other microtubule-associated proteins, motor proteins utilise the energy from ATP hydrolysis to generate mechanical piece of work that moves the protein along the substrate. The major motor proteins that interact with microtubules are kinesin, which ordinarily moves toward the (+) cease of the microtubule, and dynein, which moves toward the (−) stop.
- Dynein is equanimous of two identical heavy chains, which make up 2 large globular head domains, and a variable number of intermediate and light chains. Dynein-mediated transport takes place from the (+) end towards the (-) end of the microtubule. ATP hydrolysis occurs in the globular caput domains, which share similarities with the AAA+ (ATPase associated with diverse cellular activities) protein family unit. ATP hydrolysis in these domains is coupled to movement along the microtubule via the microtubule-binding domains. Dynein transports vesicles and organelles throughout the cytoplasm. In order to do this, dynein molecules bind organelle membranes via a poly peptide complex that contains a number of elements including dynactin.
- Kinesin has a similar structure to dynein. Kinesin is involved in the transport of a multifariousness of intracellular cargoes, including vesicles, organelles, protein complexes, and mRNAs toward the microtubule's (+) end.[51]
Some viruses (including retroviruses, herpesviruses, parvoviruses, and adenoviruses) that require access to the nucleus to replicate their genomes attach to motor proteins.
Mitosis [edit]
Centrosomes [edit]

A 3D diagram of a centriole. Each circumvolve represents 1 microtubule. In total there are 27 microtubules organized into 9 bundles of three.
The centrosome is the master MTOC (microtubule organizing center) of the cell during mitosis. Each centrosome is fabricated up of 2 cylinders called centrioles, oriented at right angles to each other. The centriole is formed from 9 main microtubules, each having two partial microtubules fastened to it. Each centriole is approximately 400 nm long and around 200 nm in circumference.[52]
The centrosome is critical to mitosis as most microtubules involved in the procedure originate from the centrosome. The minus ends of each microtubule begin at the centrosome, while the plus ends radiate out in all directions. Thus the centrosome is also of import in maintaining the polarity of microtubules during mitosis.[53]
Nigh cells simply take 1 centrosome for near of their jail cell cycle, however, right earlier mitosis, the centrosome duplicates, and the cell contains ii centrosomes.[54] Some of the microtubules that radiate from the centrosome grow direct away from the sis centrosome. These microtubules are called astral microtubules. With the assistance of these astral microtubules the centrosomes move away from each other towards contrary sides of the cell. One time there, other types of microtubules necessary for mitosis, including interpolar microtubules and K-fibers can brainstorm to form.[55]
A final of import note almost the centrosomes and microtubules during mitosis is that while the centrosome is the MTOC for the microtubules necessary for mitosis, inquiry has shown that one time the microtubules themselves are formed and in the correct identify the centrosomes themselves are not needed for mitosis to occur.[56]
Microtubule subclasses [edit]
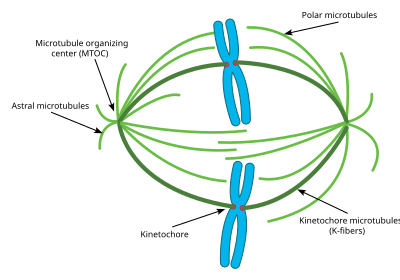
This diagram depicts the organization of a typical mitotic spindle establish in animal cells. Shown here are the iii master types of microtubules during mitosis and how they are oriented in the cell and the mitotic spindle.
Astral microtubules are a subclass of microtubules which only exist during and around mitosis. They originate from the centrosome, only do not interact with the chromosomes, kinetochores, or with the microtubules originating from the other centrosome.[57] Instead their microtubules radiate towards the cell membrane. Once there they interact with specific motor proteins which create forcefulness that pull the microtubules, and thus the unabridged centrosome towards the cell membrane. As stated above, this helps the centrosomes orient themselves away from each other in the cell. Yet these astral microtubules practice non interact with the mitotic spindle itself. Experiments accept shown that without these astral microtubules, the mitotic spindle tin form, however its orientation in the prison cell is not always correct and thus mitosis does not occur every bit finer.[58] Another key part of the astral microtubules is to assistance in cytokinesis. Astral microtubules interact with motor proteins at the cell membrane to pull the spindle and the entire cell apart once the chromosomes take been replicated.
Interpolar/Polar microtubules are a class of microtubules which also radiate out from the centrosome during mitosis. These microtubules radiate towards the mitotic spindle, dissimilar astral microtubules. Interpolar microtubules are both the well-nigh abundant and dynamic bracket of microtubules during mitosis. Around 95 percent of microtubules in the mitotic spindle can be characterized as interpolar. Furthermore, the half life of these microtubules is extremely short as it is less than one minute.[59] Interpolar microtubules that do not attach to the kinetochores can assistance in chromosome congregation through lateral interaction with the kinetochores.[60]
K fibers/Kinetochore microtubules are the third of import bracket of mitotic microtubules. These microtubules form direct connections with the kinetochores in the mitotic spindle. Each K fiber is composed of xx–xl parallel microtubules, forming a strong tube which is attached at ane end to the centrosome and on the other to the kinetochore, located in the center of each chromosome. Since each centrosome has a K fiber connecting to each pair of chromosomes, the chromosomes go tethered in the eye of the mitotic spindle past the K fibers. One thousand fibers have a much longer half life than interpolar microtubules, at between iv and eight minutes.[61] During the finish of mitoses, the microtubules forming each K cobweb brainstorm to disassociate, thus shorting the One thousand fibers. As the Grand fibers shorten the pair chromosomes are pulled apart correct before cytokinesis. Previously, some researchers believed that K fibers form at their minus end originating from the centrosome just similar other microtubules, however, new inquiry has pointed to a different mechanism. In this new mechanism, the K fibers are initially stabilized at their plus end by the kinetochores and grow out from there. The minus finish of these K fibers eventually connect to an existing Interpolar microtubule and are eventually connected to the centrosome in this fashion.[62]
Microtubule nuclear in the mitotic spindle [edit]
Virtually of the microtubules that class the mitotic spindle originate from the centrosome. Originally information technology was thought that all of these microtubules originated from the centrosome via a method called search and capture, described in more particular in a section above, however new research has shown that there are addition means of microtubule nucleation during mitosis. I of the most important of these additional means of microtubule nucleation is the RAN-GTP pathway. RAN-GTP associates with chromatin during mitosis to create a slope that allows for local nucleation of microtubules almost the chromosomes. Furthermore, a second pathway known as the augmin/HAUS complex (some organisms apply the more studied augmin circuitous, while others such as humans use an analogous circuitous called HAUS) acts an additional means of microtubule nucleation in the mitotic spindle.[62]
Functions [edit]
Prison cell migration [edit]
Microtubule plus ends are often localized to particular structures. In polarized interphase cells, microtubules are unduly oriented from the MTOC toward the site of polarity, such every bit the leading edge of migrating fibroblasts. This configuration is thought to help deliver microtubule-bound vesicles from the Golgi to the site of polarity.
Dynamic instability of microtubules is also required for the migration of most mammalian cells that crawl.[63] Dynamic microtubules regulate the levels of key One thousand-proteins such as RhoA[64] and Rac1,[65] which regulate prison cell contractility and cell spreading. Dynamic microtubules are also required to trigger focal adhesion disassembly, which is necessary for migration.[66] It has been constitute that microtubules human action as "struts" that counteract the contractile forces that are needed for trailing edge retraction during cell move. When microtubules in the abaft edge of cell are dynamic, they are able to remodel to allow retraction. When dynamics are suppressed, microtubules cannot remodel and, therefore, oppose the contractile forces.[46] The morphology of cells with suppressed microtubule dynamics indicate that cells can extend the forepart edge (polarized in the direction of movement), but have difficulty retracting their trailing border.[67] On the other hand, high drug concentrations, or microtubule mutations that depolymerize the microtubules, tin restore prison cell migration but at that place is a loss of directionality. It can be ended that microtubules act both to restrain cell movement and to plant directionality
Cilia and flagella [edit]
Microtubules have a major structural role in eukaryotic cilia and flagella. Cilia and flagella ever extend directly from a MTOC, in this case termed the basal body. The action of the dynein motor proteins on the diverse microtubule strands that run forth a cilium or flagellum allows the organelle to bend and generate force for swimming, moving extracellular material, and other roles. Prokaryotes possess tubulin-like proteins including FtsZ. However, prokaryotic flagella are entirely dissimilar in structure from eukaryotic flagella and do not contain microtubule-based structures.
Development [edit]
The cytoskeleton formed by microtubules is essential to the morphogenetic process of an organism'south evolution. For example, a network of polarized microtubules is required inside the oocyte of Drosophila melanogaster during its embryogenesis in order to establish the centrality of the egg. Signals sent between the follicular cells and the oocyte (such equally factors like to epidermal growth factor) cause the reorganization of the microtubules so that their (-) ends are located in the lower part of the oocyte, polarizing the construction and leading to the advent of an anterior-posterior axis.[68] This involvement in the body'due south architecture is also seen in mammals.[69]
Some other area where microtubules are essential is the evolution of the nervous system in higher vertebrates, where tubulin'due south dynamics and those of the associated proteins (such as the microtubule-associated proteins) is finely controlled during the evolution of the nervous organization.[70]
Cistron regulation [edit]
The cellular cytoskeleton is a dynamic system that functions on many different levels: In addition to giving the cell a particular form and supporting the transport of vesicles and organelles, it can besides influence gene expression. The signal transduction mechanisms involved in this communication are trivial understood. However, the relationship between the drug-mediated depolymerization of microtubules, and the specific expression of transcription factors has been described, which has provided information on the differential expression of the genes depending on the presence of these factors.[71] This communication between the cytoskeleton and the regulation of the cellular response is besides related to the action of growth factors: for example, this relation exists for connective tissue growth factor.[72]
See besides [edit]
- Microtentacle
- Orchestrated objective reduction – a hypothesis explaining consciousness
References [edit]
- ^ "Digital Downloads". PurSolutions . Retrieved 2020-02-20 .
- ^ Ledbetter MC, Porter KR (1963). "A "microtubule" in plant cell fine construction". Journal of Jail cell Biology. 19 (ane): 239–50. doi:10.1083/jcb.19.i.239. PMC2106853. PMID 19866635.
- ^ Chalfie Thousand, Thomson JN (1979). "Organization of neuronal microtubules in the nematode Caenorhabditis elegans". Journal of Cell Biological science. 82 (1): 278–89. doi:ten.1083/jcb.82.ane.278. PMC2110421. PMID 479300.
- ^ Diwan JJ (2006). "Microtubules". Rensselaer Polytechnic Establish. Archived from the original on 2014-02-06. Retrieved 2014-02-24 .
- ^ Vale RD (February 2003). "The molecular motor toolbox for intracellular ship". Cell. 112 (4): 467–80. doi:ten.1016/S0092-8674(03)00111-9. PMID 12600311. S2CID 15100327.
- ^ Howard J, Hyman AA (February 2007). "Microtubule polymerases and depolymerases". Current Opinion in Jail cell Biology. 19 (1): 31–v. doi:10.1016/j.ceb.2006.12.009. PMID 17184986.
- ^ Jiang Southward, Narita A, Popp D, Ghoshdastider U, Lee LJ, Srinivasan R, Balasubramanian MK, Oda T, Koh F, Larsson 1000, Robinson RC (March 2016). "Novel actin filaments from Bacillus thuringiensis form nanotubules for plasmid Deoxyribonucleic acid segregation". Proceedings of the National Academy of Sciences of the Us of America. 113 (9): E1200-5. Bibcode:2016PNAS..113E1200J. doi:ten.1073/pnas.1600129113. PMC4780641. PMID 26873105.
- ^ Wayne, R. 2009. Plant Jail cell Biological science: From Astronomy to Zoology. Amsterdam: Elsevier/Academic Press, p. 165.
- ^ Cooper GM (2000). "Microtubule Motors and Movements". The Cell: A Molecular Approach. 2nd Edition . Retrieved 2019-03-12 .
- ^ Kapoor V, Hirst WG, Hentschel C, Preibisch S, Reber South (March 2019). "MTrack: Automated Detection, Tracking, and Analysis of Dynamic Microtubules". Scientific Reports. 9 (ane): 3794. Bibcode:2019NatSR...9.3794K. doi:10.1038/s41598-018-37767-1. PMC6405942. PMID 30846705.
- ^ Löwe J, Li H, Downing KH, Nogales East (November 2001). "Refined structure of alpha beta-tubulin at three.five A resolution". Periodical of Molecular Biology. 313 (5): 1045–57. doi:10.1006/jmbi.2001.5077. PMID 11700061.
- ^ a b c Weisenberg RC (September 1972). "Microtubule germination in vitro in solutions containing depression calcium concentrations". Science. 177 (4054): 1104–5. Bibcode:1972Sci...177.1104W. doi:x.1126/science.177.4054.1104. PMID 4626639. S2CID 34875893.
- ^ Desai A, Mitchison TJ (1997). "Microtubule polymerization dynamics". Annual Review of Jail cell and Developmental Biology. 13: 83–117. doi:10.1146/annurev.cellbio.13.one.83. PMID 9442869.
- ^ Desai, A.; Mitchison, T. J. (1997). "Microtubule polymerization dynamics". Annual Review of Cell and Developmental Biological science. 13: 83–117. doi:10.1146/annurev.cellbio.13.ane.83. ISSN 1081-0706. PMID 9442869.
- ^ Chaaban S, Brouhard GJ (2017). "A microtubule bestiary: structural diversity in tubulin polymers". Molecular Biological science of the Jail cell. 28 (22): 2924–31. doi:10.1091/mbc.E16-05-0271. PMC5662251. PMID 29084910.
- ^ Chrétien D, Metoz F, Verde F, Karsenti E, Wade RH (June 1992). "Lattice defects in microtubules: protofilament numbers vary within individual microtubules". Periodical of Jail cell Biology. 117 (five): 1031–40. doi:x.1083/jcb.117.v.1031. PMC2289483. PMID 1577866.
- ^ Walker RA, O'Brien ET, Pryer NK, Soboeiro MF, Voter WA, Erickson HP, Salmon ED (October 1988). "Dynamic instability of individual microtubules analyzed by video low-cal microscopy: charge per unit constants and transition frequencies". The Journal of Cell Biology. 107 (4): 1437–48. CiteSeerXten.1.i.525.507. doi:10.1083/jcb.107.iv.1437. PMC2115242. PMID 3170635.
- ^ Sui H, Downing KH (August 2010). "Structural basis of interprotofilament interaction and lateral deformation of microtubules". Structure. 18 (8): 1022–31. doi:10.1016/j.str.2010.05.010. PMC2976607. PMID 20696402.
- ^ Bassen DM, Hou Y, Bowser SS, Banavali NK (August 2016). "Maintenance of electrostatic stabilization in altered tubulin lateral contacts may facilitate germination of helical filaments in foraminifera". Scientific Reports. vi: 31723. Bibcode:2016NatSR...631723B. doi:10.1038/srep31723. PMC4990898. PMID 27539392.
- ^ Nogales E (2000). "Structural insights into microtubule role". Almanac Review of Biochemistry. 69: 277–302. doi:10.1146/annurev.biochem.69.ane.277. PMID 10966460.
- ^ Schlieper D, Oliva MA, Andreu JM, Löwe J (June 2005). "Structure of bacterial tubulin BtubA/B: show for horizontal gene transfer". Proceedings of the National Academy of Sciences of the Us of America. 102 (26): 9170–5. Bibcode:2005PNAS..102.9170S. doi:10.1073/pnas.0502859102. PMC1166614. PMID 15967998.
- ^ Pilhofer M, Ladinsky MS, McDowall AW, Petroni Chiliad, Jensen GJ (December 2011). "Microtubules in leaner: Ancient tubulins build a v-protofilament homolog of the eukaryotic cytoskeleton". PLOS Biology. 9 (12): e1001213. doi:ten.1371/periodical.pbio.1001213. PMC3232192. PMID 22162949.
- ^ "Medical Definition of Neurotubules". www.merriam-webster.com.
- ^ Zhao B, Meka DP, Scharrenberg R, König T, Schwanke B, Kobler O, Windhorst S, Kreutz MR, Mikhaylova M, Calderon de Anda F (Baronial 2017). "Microtubules Modulate F-actin Dynamics during Neuronal Polarization". Scientific Reports. vii (one): 9583. Bibcode:2017NatSR...vii.9583Z. doi:ten.1038/s41598-017-09832-8. PMC5575062. PMID 28851982.
- ^ Bartolini F, Gundersen GG (October 2006). "Generation of noncentrosomal microtubule arrays". Journal of Jail cell Science. 119 (Pt 20): 4155–63. doi:10.1242/jcs.03227. PMID 17038542.
- ^ Desai A, Mitchison TJ (1997). "Microtubule polymerization dynamics". Annual Review of Cell and Developmental Biology. 13: 83–117. doi:10.1146/annurev.cellbio.thirteen.1.83. PMID 9442869.
- ^ Vinogradova T, Miller PM, Kaverina I (July 2009). "Microtubule network disproportion in motile cells: function of Golgi-derived assortment". Cell Bike. 8 (14): 2168–74. doi:10.4161/cc.8.14.9074. PMC3163838. PMID 19556895.
- ^ Uehara R, Nozawa RS, Tomioka A, Petry S, Vale RD, Obuse C, Goshima Thousand (April 2009). "The augmin complex plays a critical role in spindle microtubule generation for mitotic progression and cytokinesis in human cells". Proceedings of the National University of Sciences of the United States of America. 106 (17): 6998–7003. Bibcode:2009PNAS..106.6998U. doi:x.1073/pnas.0901587106. PMC2668966. PMID 19369198.
- ^ Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002). "The Self-Associates and Dynamic Construction of Cytoskeletal Filaments". Molecular Biology of the Prison cell (4th ed.). New York: Garland Science.
- ^ Karp Chiliad (2005). Prison cell and Molecular Biology: Concepts and Experiments. Usa: John Wiley & Sons. p. 355. ISBN978-0-471-46580-5.
- ^ Weisenberg RC, Deery WJ, Dickinson PJ (September 1976). "Tubulin-nucleotide interactions during the polymerization and depolymerization of microtubules". Biochemistry. fifteen (19): 4248–54. doi:10.1021/bi00664a018. PMID 963034.
- ^ Mitchison T, Kirschner Grand (1984). "Dynamic instability of microtubule growth". Nature. 312 (5991): 237–42. Bibcode:1984Natur.312..237M. doi:ten.1038/312237a0. PMID 6504138. S2CID 30079133.
- ^ Kirschner M, Mitchison T (May 1986). "Across self-assembly: from microtubules to morphogenesis". Jail cell. 45 (three): 329–42. doi:10.1016/0092-8674(86)90318-1. PMID 3516413. S2CID 36994346.
- ^ Cheeseman IM, Desai A (Jan 2008). "Molecular architecture of the kinetochore-microtubule interface". Nature Reviews. Molecular Cell Biology. 9 (i): 33–46. doi:x.1038/nrm2310. PMID 18097444. S2CID 34121605.
- ^ a b Infante AS, Stein MS, Zhai Y, Borisy GG, Gundersen GG (November 2000). "Detyrosinated (Glu) microtubules are stabilized past an ATP-sensitive plus-end cap". Periodical of Jail cell Science. 113 (22): 3907–19. doi:10.1242/jcs.113.22.3907. PMID 11058078.
- ^ Palazzo AF, Cook TA, Alberts AS, Gundersen GG (August 2001). "mDia mediates Rho-regulated formation and orientation of stable microtubules". Nature Prison cell Biological science. iii (8): 723–9. doi:ten.1038/35087035. PMID 11483957. S2CID 7374170.
- ^ Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch Due north, Morris EJ, Chen M, Wallar BJ, Alberts As, Gundersen GG (September 2004). "EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration". Nature Cell Biology. six (9): 820–30. doi:10.1038/ncb1160. PMID 15311282. S2CID 29214110.
- ^ a b Janke C, Bulinski JC (Nov 2011). "Postal service-translational regulation of the microtubule cytoskeleton: mechanisms and functions". Nature Reviews. Molecular Cell Biology. 12 (12): 773–86. doi:10.1038/nrm3227. PMID 22086369. S2CID 5969290.
- ^ a b c Garnham CP, Roll-Mecak A (July 2012). "The chemical complexity of cellular microtubules: tubulin post-translational modification enzymes and their roles in tuning microtubule functions". Cytoskeleton. 69 (7): 442–63. doi:10.1002/cm.21027. PMC3459347. PMID 22422711.
- ^ Ersfeld K, Wehland J, Plessmann U, Dodemont H, Gerke V, Weber K (February 1993). "Label of the tubulin-tyrosine ligase". The Journal of Prison cell Biology. 120 (3): 725–32. doi:ten.1083/jcb.120.iii.725. PMC2119537. PMID 8093886.
- ^
- ^ Kalebic North, Sorrentino South, Perlas E, Bolasco G, Martinez C, Heppenstall PA (2013-06-ten). "αTAT1 is the major α-tubulin acetyltransferase in mice". Nature Communications. 4: 1962. Bibcode:2013NatCo...4.1962K. doi:10.1038/ncomms2962. PMID 23748901.
- ^ Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP (May 2002). "HDAC6 is a microtubule-associated deacetylase". Nature. 417 (6887): 455–8. Bibcode:2002Natur.417..455H. doi:ten.1038/417455a. PMID 12024216. S2CID 4373254.
- ^ Audebert South, Desbruyères Eastward, Gruszczynski C, Koulakoff A, Gros F, Denoulet P, Eddé B (June 1993). "Reversible polyglutamylation of alpha- and beta-tubulin and microtubule dynamics in mouse encephalon neurons". Molecular Biological science of the Cell. 4 (half dozen): 615–26. doi:10.1091/mbc.4.half dozen.615. PMC300968. PMID 8104053.
- ^ Ganguly A, Yang H, Cabral F (November 2010). "Paclitaxel-dependent prison cell lines reveal a novel drug activity". Molecular Cancer Therapeutics. 9 (eleven): 2914–23. doi:ten.1158/1535-7163.MCT-10-0552. PMC2978777. PMID 20978163.
- ^ a b Yang H, Ganguly A, Cabral F (Oct 2010). "Inhibition of cell migration and cell division correlates with singled-out effects of microtubule inhibiting drugs". The Journal of Biological Chemistry. 285 (42): 32242–50. doi:ten.1074/jbc.M110.160820. PMC2952225. PMID 20696757.
- ^ Burgess J, Northcote DH (September 1969). "Action of colchicine and heavy h2o on the polymerization of microtubules in wheat root meristem". Journal of Cell Scientific discipline. 5 (ii): 433–51. doi:10.1242/jcs.5.2.433. PMID 5362335.
- ^ Mandelkow Eastward, Mandelkow EM (Feb 1995). "Microtubules and microtubule-associated proteins". Current Opinion in Jail cell Biological science. 7 (ane): 72–81. doi:10.1016/0955-0674(95)80047-6. PMID 7755992.
- ^ Bramblett GT, Goedert Chiliad, Jakes R, Merrick SE, Trojanowski JQ, Lee VM (June 1993). "Abnormal tau phosphorylation at Ser396 in Alzheimer'south affliction recapitulates evolution and contributes to reduced microtubule binding". Neuron. 10 (vi): 1089–99. doi:10.1016/0896-6273(93)90057-X. PMID 8318230. S2CID 23180847.
- ^ "The Human Protein Atlas". www.proteinatlas.org. Archived from the original on 2017-05-01. Retrieved 2017-04-27 .
- ^ Hirokawa Northward, Noda Y, Tanaka Y, Niwa S (October 2009). "Kinesin superfamily motor proteins and intracellular transport". Nature Reviews. Molecular Cell Biology. 10 (10): 682–96. doi:10.1038/nrm2774. PMID 19773780. S2CID 18129292.
- ^ Marshall WF, Rosenbaum JL (March 1999). "Cell division: The renaissance of the centriole". Electric current Biological science. nine (half dozen): R218–xx. doi:10.1016/s0960-9822(99)80133-10. PMID 10209087. S2CID 16951268.
- ^ Pereira G, Schiebel E (February 1997). "Centrosome-microtubule nucleation". Journal of Cell Science. 110 (Pt three): 295–300. doi:10.1242/jcs.110.3.295. PMID 9057082.
- ^ Hinchcliffe EH, Sluder G (May 2001). ""Information technology takes two to tango": understanding how centrosome duplication is regulated throughout the cell cycle". Genes & Development. xv (x): 1167–81. doi:10.1101/gad.894001. PMID 11358861.
- ^ Along South, Kapoor TM (June 2017). "The mechanics of microtubule networks in cell division". The Periodical of Cell Biological science. 216 (6): 1525–1531. doi:10.1083/jcb.201612064. PMC5461028. PMID 28490474.
- ^ Khodjakov, A., Cole, R. West., Oakley, B. R. and Rieder, C. L. (2000). "Centrosome-contained mitotic spindle formation in vertebrates". Curr. Biol. ten, 59–67. doi:10.1016/S0960-9822(99)00276-6.
- ^ Rosenblatt J (March 2005). "Spindle assembly: asters part their separate ways". Nature Cell Biological science. vii (3): 219–22. doi:10.1038/ncb0305-219. PMID 15738974. S2CID 8082479.
- ^ Knoblich JA (December 2010). "Asymmetric cell division: recent developments and their implications for tumour biology". Nature Reviews. Molecular Cell Biological science. 11 (12): 849–sixty. doi:x.1038/nrm3010. PMC3941022. PMID 21102610.
- ^ Zhai Y, Kronebusch PJ, Borisy GG (Nov 1995). "Kinetochore microtubule dynamics and the metaphase-anaphase transition". The Journal of Prison cell Biology. 131 (3): 721–34. doi:ten.1083/jcb.131.3.721. PMC2120628. PMID 7593192.
- ^ Cai S, O'Connell CB, Khodjakov A, Walczak CE (July 2009). "Chromosome congression in the absenteeism of kinetochore fibres". Nature Cell Biology. eleven (7): 832–8. doi:x.1038/ncb1890. PMC2895821. PMID 19525938.
- ^ Bakhoum SF, Thompson SL, Manning AL, Compton DA (Jan 2009). "Genome stability is ensured by temporal control of kinetochore-microtubule dynamics". Nature Jail cell Biology. 11 (one): 27–35. doi:10.1038/ncb1809. PMC2614462. PMID 19060894.
- ^ a b Meunier Southward, Vernos I (June 2012). "Microtubule assembly during mitosis - from distinct origins to distinct functions?". Journal of Cell Scientific discipline. 125 (Pt 12): 2805–xiv. doi:x.1242/jcs.092429. PMID 22736044.
- ^ Mikhailov A, Gundersen GG (1998). "Relationship between microtubule dynamics and lamellipodium formation revealed past direct imaging of microtubules in cells treated with nocodazole or taxol". Cell Motility and the Cytoskeleton. 41 (four): 325–40. doi:10.1002/(SICI)1097-0169(1998)41:4<325::Help-CM5>iii.0.CO;ii-D. PMID 9858157.
- ^ Ren XD, Kiosses WB, Schwartz MA (February 1999). "Regulation of the small GTP-binding protein Rho by prison cell adhesion and the cytoskeleton". The EMBO Periodical. 18 (iii): 578–85. doi:ten.1093/emboj/18.3.578. PMC1171150. PMID 9927417.
- ^ Waterman-Storer CM, Worthylake RA, Liu BP, Burridge K, Salmon ED (May 1999). "Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts". Nature Cell Biology. 1 (ane): 45–50. doi:ten.1038/9018. PMID 10559863. S2CID 26321103.
- ^ Ezratty EJ, Partridge MA, Gundersen GG (June 2005). "Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase". Nature Jail cell Biology. 7 (six): 581–ninety. doi:10.1038/ncb1262. PMID 15895076. S2CID 37153935.
- ^ Ganguly A, Yang H, Sharma R, Patel KD, Cabral F (December 2012). "The office of microtubules and their dynamics in cell migration". The Periodical of Biological Chemistry. 287 (52): 43359–69. doi:x.1074/jbc.M112.423905. PMC3527923. PMID 23135278.
- ^ van Eeden F, St Johnston D (August 1999). "The polarisation of the anterior-posterior and dorsal-ventral axes during Drosophila oogenesis". Current Opinion in Genetics & Development. 9 (4): 396–404. doi:10.1016/S0959-437X(99)80060-4. PMID 10449356.
- ^ Beddington RS, Robertson EJ (Jan 1999). "Axis development and early asymmetry in mammals". Cell. 96 (2): 195–209. doi:ten.1016/S0092-8674(00)80560-seven. PMID 9988215. S2CID 16264083.
- ^ Tucker RP (1990). "The roles of microtubule-associated proteins in brain morphogenesis: a review". Encephalon Research. Brain Research Reviews. 15 (2): 101–20. doi:10.1016/0165-0173(90)90013-E. PMID 2282447. S2CID 12641708.
- ^ Rosette C, Karin G (March 1995). "Cytoskeletal control of gene expression: depolymerization of microtubules activates NF-kappa B". The Journal of Cell Biology. 128 (6): 1111–ix. doi:10.1083/jcb.128.6.1111. PMC2120413. PMID 7896875.
- ^ Ott C, Iwanciw D, Graness A, Giehl M, Goppelt-Struebe M (Nov 2003). "Modulation of the expression of connective tissue growth factor by alterations of the cytoskeleton". The Journal of Biological Chemistry. 278 (45): 44305–11. doi:ten.1074/jbc.M309140200. PMID 12951326.
External links [edit]
- MBInfo - Microtubules
- 3D microtubule structures in the EM Information Banking concern(EMDB)
- Protocols for generating microtubules
Source: https://en.wikipedia.org/wiki/Microtubule
Posted by: stricklandwhousen.blogspot.com

0 Response to "What Does The Microtubule Do In An Animal Cell"
Post a Comment